Can Hydrogen Peroxide Be Used to Remove Manganese and Iron from well water?
Recent studies have looked at the effectiveness of different methods of removing manganese from well water. A thorough study on this topic was done by a graduate student at the University of Pittsburgh.
The project worked to figure out the optimal treatment method as a result of which the largest amount of manganese was removed and the smallest amount of disinfectant byproduct was left over. To break down ions such as manganese and iron, an appropriate injectant must be used to reduce the number of electrons each ion has.
To break down ions such as manganese and iron, an appropriate injectant must be used to reduce the number of electrons each ion has.
The substance that receives the extra electrons is called the oxidizing agent.
The study found that “Hydrogen Peroxide at these [varying] pH levels did not prove to be an adequate oxidizing agent for manganese.
Iron however was oxidized when hydrogen peroxide was present…
In conclusion, there was no benefit to using hydrogen peroxide. Peroxide did not oxidize the manganese and it created massive complications with chlorine used for disinfection.
The chart above shows the manganese removal results from different injectants used. One interesting point about the chart, is that it takes over 100 minutes with chlorine to perfectly oxidize manganese.
But if chlorine combined with our Pro-OX manganese dioxide media iron filters, or Greensand iron filters, the manganese is effectively removed by a catalytic reaction between the filter media and the chlorine, which results in manganese getting instantly oxidized on the surface of the filter media. A strong backwash (10 GPM is OK) is needed to flush out the accumulated manganese oxides.
Therefore, the Pro-Ox iron filter with manganese dioxide media should be paired with a chlorine feed for most effective manganese removal.

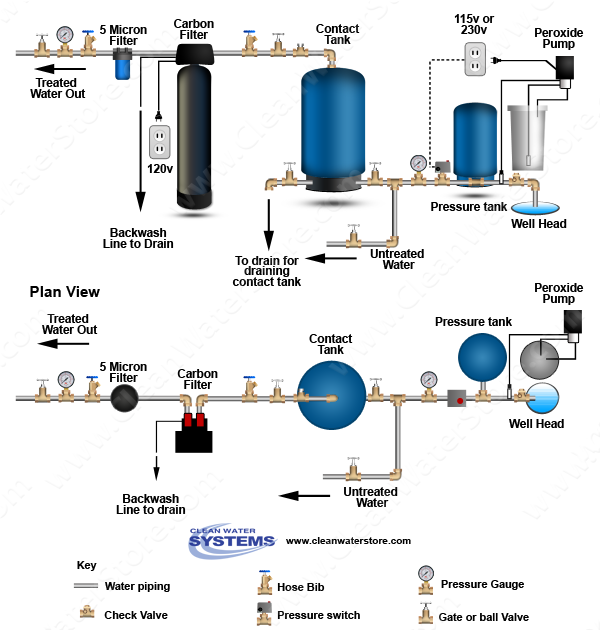
Below is a diagram showing the way the system is set up.
Follow this link to see the full thesis.